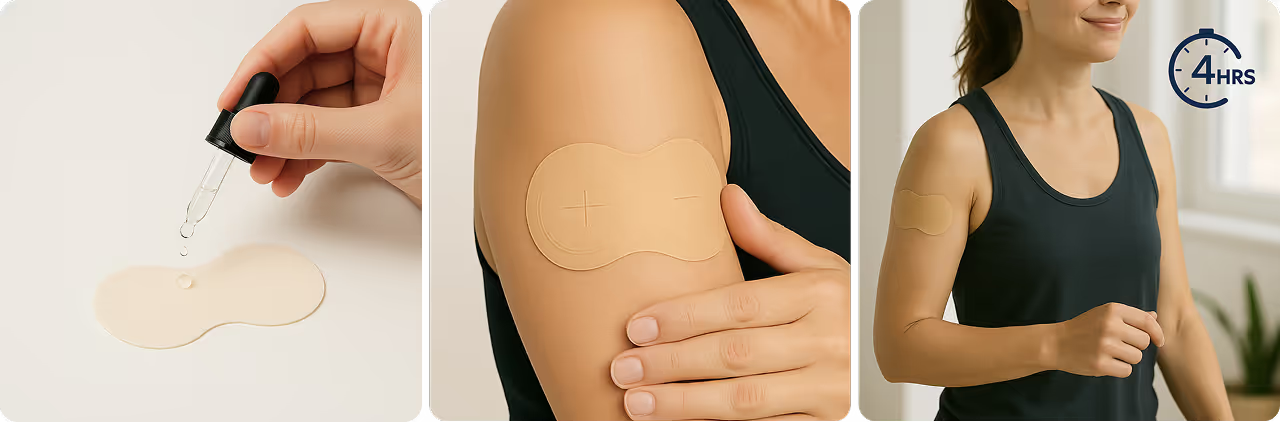
Storage
Always refrigerate your vial at 36–46 °F (2–8 °C), never freeze it, and avoid storing it next to the cooling element. Protect your vials from direct sunlight and heat.
Instructions
This information is intended for individuals who have been prescribed Tirzepatide Injections by their Alan Health provider.
Inject once weekly as directed by your healthcare provider using a vial and syringe.
{{injection-video}}
Clean the injection site, insert the needle at a 45–90-degree angle, and inject the medication slowly. Hold until fully administered. Rotate injection sites daily to avoid irritation.
- Prep Your Supplies: Wash hands and gather the tirzepatide vial, insulin syringe, alcohol swabs, and a sharps container. Inspect the solution—it should be clear and free of particles.
- Draw the Dose: Clean the rubber top of the vial with an alcohol swab. Inject air into the vial equal to your prescribed dose, then draw up the exact amount. Remove any air bubbles by tapping the syringe and pushing gently on the plunger.
- Pick an Injection Site: Use the abdomen (at least 2 inches from the belly button), thigh, or upper arm. Clean the area with an alcohol swab and allow it to dry completely.
- Inject: Gently pinch the skin. Insert the needle at a 45–90° angle, depending on needle length and body type. Inject the medication slowly and steadily. Once done, remove the needle and apply gentle pressure with a clean swab. Dispose of the syringe safely in a sharps container.
If your medication has a seal, watch this video to learn how to remove it.
To learn how to inject your medication subcutaneously, please watch this video.
Missed Dose? If you miss a dose, skip the missed dose and continue with your regular schedule. If you have any questions about your dosing schedule, contact your Alan Health provider.
Understanding your insulin syringe
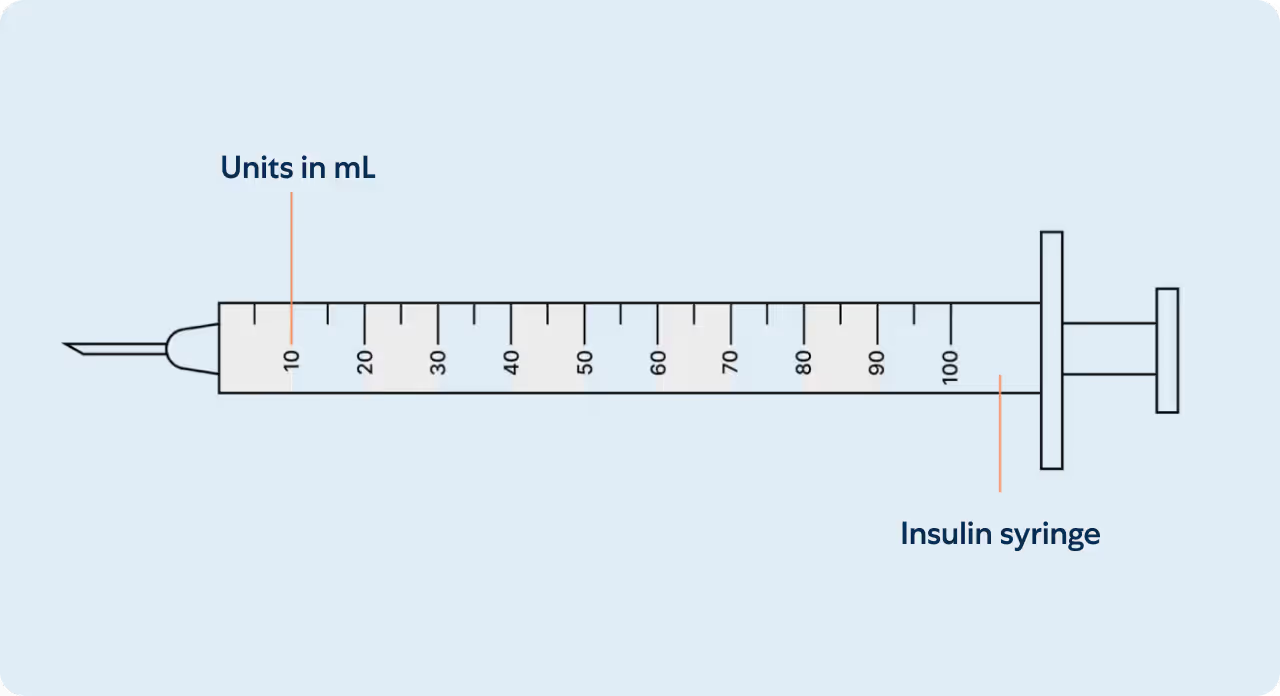
Measuring your dose
Insulin syringes are used for subcutaneous injections. They are marked in Units on the insulin syringe barrel (see illustration). Insulin syringes facilitate the precise measurement of tiny amounts of liquids which are ideal for medications such as insulin that require small and accurate dosing. Unit marks are also expressed in mL’s and can be interchangeably referenced. A prescriber may write a prescription in Units or in mL’s.
Within the amount of liquid is the prescribed mg dosage of your medication (see mg chart). Milligrams (mg) is not an amount of liquid, but rather the amount of drug that is within. The conversion between units and milligrams varies depending on the concentration of the product being used.
What can I expect?
Safety information
The most common side effects of tirzepatide include nausea, vomiting, and diarrhea.
In rare cases, side effects include pancreatitis and thyroid tumors. Seek immediate medical care if these occur.
A full list of tirzepatide side effects can be found here.
Vitamin B6 injections correct deficiencies that can cause anemia, skin changes, mood issues, confusion, seizures, and nerve problems. Side effects are rare at recommended doses, but long-term high doses may cause reversible sensory nerve damage (numbness, tingling), with dizziness and skin rash being uncommon. Report any such symptoms to your primary physician immediately.
Indications
A full list of Vitamin B6 side effects can be found here.
Tirzepatide is a prescription glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist indicated:
- As an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults with a body mass index (BMI) ≥30 kg/m² (obesity), or ≥27 kg/m² (overweight) in the presence of at least one weight-related comorbidity (e.g., hypertension, type 2 diabetes, or dyslipidemia).
BLACK BOX WARNING: Risk of Thyroid C-Cell Tumors
In animal studies, tirzepatide caused thyroid C-cell tumors, including medullary thyroid carcinoma (MTC). It is unknown whether tirzepatide causes thyroid C-cell tumors, including MTC, in humans.
Do not use tirzepatide if you:
- Have a personal or family history of MTC or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Are pregnant or planning to become pregnant without consulting your healthcare provider.
Contraindications
Do not take tirzepatide if you:
- Have a personal or family history of MTC or MEN 2.
- Have a known hypersensitivity to tirzepatide or any of its components.
- Are pregnant or planning to become pregnant.
- Are breastfeeding or planning to breastfeed.
- Have a history of pancreatitis.
- Have type 1 diabetes mellitus.
- Have severe gastrointestinal disease, including gastroparesis or intestinal obstruction.
- Are currently taking another GLP-1 receptor agonist or GIP/GLP-1 combination product.
Warnings and Precautions
- Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been reported. Discontinue if pancreatitis is suspected.
- Gallbladder Disease: Increased risk of gallstones and cholecystitis. Monitor for symptoms such as abdominal pain or jaundice.
- Hypoglycemia: When used with insulin or insulin secretagogues (e.g., sulfonylureas), hypoglycemia may occur. Monitor glucose levels closely and adjust doses accordingly.
- Renal Impairment: Cases of acute kidney injury have been reported, usually in the context of gastrointestinal side effects like vomiting and diarrhea. Monitor renal function, especially in susceptible individuals.
- Diabetic Retinopathy Complications: Rapid improvements in glucose control may worsen retinopathy. Monitor patients with pre-existing retinopathy.
- Heart Rate Increase: An increase in resting heart rate has been observed. Monitor periodically.
- Suicidal Behavior and Ideation: Monitor for depression, mood changes, or suicidal thoughts. Discontinue if such symptoms occur.
- Hypersensitivity Reactions: Severe allergic reactions, including anaphylaxis and angioedema, have occurred. Discontinue immediately if hypersensitivity is suspected.
- Aspiration Risk: Like other GLP-1s, tirzepatide may increase the risk of aspiration during anesthesia. Inform the surgical team prior to procedures.
Drug Interactions and Limitations of Use
- Tirzepatide slows gastric emptying and may affect the absorption of oral medications. Monitor when co-administering with oral drugs requiring rapid absorption.
- Do not use in combination with other GLP-1 receptor agonists or GIP/GLP-1 receptor agonists.
- Vitamin B6 may interact with certain medications, including some chemotherapy drugs (e.g., altretamine), sedatives (barbiturates), seizure medications (fosphenytoin, phenytoin), and levodopa for Parkinson’s disease. Consult your healthcare provider before use.
Reproductive Considerations
- Pregnancy: Tirzepatide should be discontinued at least 1 month prior to a planned pregnancy due to its half-life and washout period. Weight loss during pregnancy is not recommended.
- Breastfeeding: Avoid use while breastfeeding unless specifically advised by a healthcare provider.
To Report Adverse Reactions, Contact the FDA:
- Phone: 1-800-FDA-1088
- Online: www.fda.gov/medwatch
Disclaimer
These statements are intended for informational purposes and do not replace professional medical advice. Always consult your healthcare provider with any questions about tirzepatide or your medical condition.
Note: The above statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Frequently Asked Questions
What is Compounded Tirzepatide+ and how does it work?
Tirzepatide is a prescription medication that supports weight loss and helps regulate blood sugar. It works by activating two natural hormone pathways—GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide)—to help reduce appetite, slow digestion, and improve how your body processes sugar and fat.
What makes Compounded Tirzepatide+ unique is that it is compounded with additional ingredients that may further support your health goals. Depending on your prescription, Tirzepatide+ may include:
- B6 – may help ease nausea
- B12 – supports energy and metabolism
- Glycine – an amino acid that supports healthy metabolism and cellular function
- NAD+ – involved in cellular energy production and healthy aging
These additions are selected by your prescribing provider based on your individual needs and treatment plan.
How often do I take Compounded Tirzepatide+?
Compounded Tirzepatide+ injections are taken once a week, ideally on the same day and time each week. Your personalized, doctor-guided plan will start at a low dose and gradually increase it to help your body adjust and minimize side effects.
How long does it take to see results?
Most users begin noticing appetite changes in the first few weeks and measurable weight loss by weeks 4–8. Optimal results often build over 3–6 months or more.
How do you ensure the quality of the medications?
We only work with FDA-registered 503B outsourcing facilities that meet the highest standards for sterility, potency, and quality. These federally regulated pharmacies are routinely inspected, batch-test all medications, and operate in tightly controlled environments.
Your safety is our priority—every pharmacy partner is carefully vetted to ensure full compliance with strict FDA standards.
What is compounded medication?
Compounding is the process of creating a medication that’s tailored to the needs of an individual patient. For example, some patients may need a medication in liquid form when the FDA-approved drug comes in a tablet. Additionally, a pharmacy can compound medications of FDA-approved drugs that are listed on the FDA’s shortage list.
Compounded drugs are prepared by state-licensed compounding pharmacies that meet federal and state requirements, including quality standards. When compounding in compliance with federal and state law, compounded drugs are not subject to FDA approval and are not evaluated for safety or efficacy.
At Alan Health, your compounded tirzepatide is made in FDA-registered 503B outsourcing facilities. These facilities meet strict federal and state requirements, follow rigorous quality and sterility standards, and are regularly inspected by the FDA. This ensures every dose is produced to the highest safety and consistency standards—while allowing for customizations that support your health and comfort.
How is this different from the tirzepatide I’ve heard about or taken before?
The biggest difference is that some Alan prescriptions are compounded with supportive additives, while others include tirzepatide alone. Your medication may include ingredients like Vitamin B6, Vitamin B12, Glycine, or NAD+, which are sometimes used to support comfort, energy, or overall tolerance during treatment.
What can I expect during the first few weeks?
You may feel fuller sooner, have less appetite, and lose some weight even in the first month. Nausea, constipation, or fatigue are common early side effects but typically improve as your body adapts.
Where do I inject Compounded Tirzepatide+?
Compounded Tirzepatide+ is injected just under the skin (subcutaneously), typically in the stomach, thigh, or back of the upper arm. Rotate sites weekly to avoid irritation.
Can I take Compounded Tirzepatide+ with other medications?
Always check with your provider. Some diabetes or blood pressure medications may need dose adjustments when you start treatment.
Do I need to follow a special diet?
Not necessarily, but eating smaller meals, staying hydrated, and avoiding greasy foods can help reduce GI side effects and improve results.
Additional support
For details about your medication and dosing, please log in to your patient portal. Your physician is here to help with any medical questions. If you are experiencing a medical emergency, call 911 right away.