Storage
Keep unopened pens refrigerated at 36–46 °F (2–8 °C). Do not freeze. After first use, you may store it at room temperature (≤ 86 °F / 30 °C) for up to 21 days—then discard. Avoid excessive heat and light exposure. For more information, visit Eli Lilly’s Zepbound site.
Instructions
This information is intended for individuals who have been prescribed Zepbound by their Alan Health provider.
For instructions on how to administer this medication, please visit the manufacturer’s website: Zepbound website
Understanding your insulin syringe
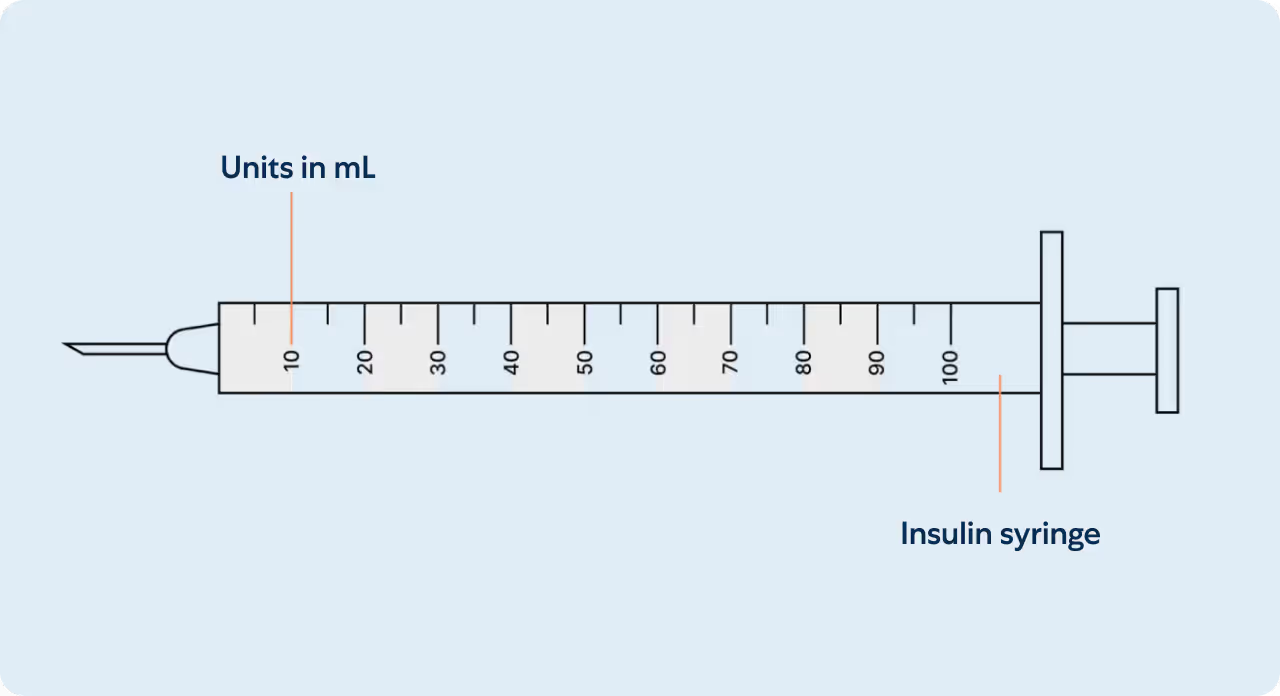
Measuring your dose
Insulin syringes are used for subcutaneous injections. They are marked in Units on the insulin syringe barrel (see illustration). Insulin syringes facilitate the precise measurement of tiny amounts of liquids which are ideal for medications such as insulin that require small and accurate dosing. Unit marks are also expressed in mL’s and can be interchangeably referenced. A prescriber may write a prescription in Units or in mL’s.
Within the amount of liquid is the prescribed mg dosage of your medication (see mg chart). Milligrams (mg) is not an amount of liquid, but rather the amount of drug that is within. The conversion between units and milligrams varies depending on the concentration of the product being used.
What can I expect?
Safety information
The most common side effects of ZEPBOUND® (tirzepatide) include nausea, diarrhea, and vomiting.
Other common side effects may include constipation, abdominal pain, indigestion (dyspepsia), injection site reactions, fatigue, hypersensitivity reactions, belching (eructation), hair loss, and gastroesophageal reflux disease. If these symptoms persist or worsen, stop taking the medication and contact your healthcare provider.
A full list of side effects can be found here.
Indications
ZEPBOUND® is a prescription medication that acts as both a GIP and GLP-1 receptor agonist. It is indicated:
- As an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in:
- Adults with obesity (BMI ≥30), or
- Adults with overweight (BMI ≥27) who also have at least one weight-related condition (e.g., hypertension, dyslipidemia, type 2 diabetes, obstructive sleep apnea, cardiovascular disease).
- To treat moderate to severe obstructive sleep apnea (OSA) in adults with obesity.
BLACK BOX WARNING: Risk of Thyroid C-Cell Tumors
In rodent studies, tirzepatide caused thyroid C-cell tumors, including medullary thyroid carcinoma (MTC). It is unknown whether this risk applies to humans.
Do not use ZEPBOUND® if you:
- Have a personal or family history of MTC or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Are pregnant or planning to become pregnant without guidance from your healthcare provider.
Contraindications
Do not take ZEPBOUND® if you:
- Have a personal or family history of medullary thyroid carcinoma (MTC) or MEN 2.
- Have type 1 diabetes.
- Have a history of pancreatitis.
- Have severe gastrointestinal problems such as gastroparesis or issues digesting food.
- Have a known allergy to tirzepatide, GLP-1 drugs, or any of the inactive ingredients in ZEPBOUND® (including sodium chloride, sodium phosphate dibasic heptahydrate, and water for injection. pH may be adjusted with hydrochloric acid or sodium hydroxide).
- Have a history of suicidal ideation or attempts.
Warnings and Precautions
- Pancreatitis: Has been reported in trials. Discontinue ZEPBOUND® if suspected and do not restart if confirmed.
- Gallbladder Disease: Acute gallbladder disease has occurred. Monitor if symptoms of cholelithiasis arise.
- Severe Gastrointestinal Disease: ZEPBOUND® has not been studied in this population and is not recommended.
- Hypoglycemia: May occur when combined with insulin or insulin secretagogues. Adjust doses as needed and educate patients on symptoms of low blood sugar.
- Kidney Injury: Monitor renal function in patients with renal impairment, especially if reporting adverse GI effects.
- Diabetic Retinopathy: Has not been studied in patients with certain forms of diabetic eye disease. Monitor those with a history of retinopathy for worsening.
- Heart Rate Increase: Has been observed with GLP-1 receptor agonists. Monitor heart rate periodically.
- Suicidal Behavior and Ideation: Monitor for new or worsening depression or suicidal thoughts. Discontinue if symptoms occur.
- Hypersensitivity: Serious allergic reactions including anaphylaxis and angioedema have been reported. Discontinue immediately if suspected.
- Pulmonary Aspiration Risk: Inform healthcare providers about planned surgeries or procedures requiring general anesthesia or deep sedation due to aspiration risk observed with GLP-1 receptor agonists.
Drug Interactions and Limitations of Use
- ZEPBOUND® delays gastric emptying, which may affect the absorption of oral medications. Use with caution, especially for drugs requiring rapid absorption.
Do not use in combination with other tirzepatide-containing medications or GLP-1 receptor agonists. - The safety and efficacy of combining ZEPBOUND® with other weight-loss therapies have not been established.
- ZEPBOUND® has not been studied in patients with a history of pancreatitis.
Reproductive Considerations
- Pregnancy: Based on animal studies, ZEPBOUND® may cause fetal harm. Discontinue immediately if pregnancy is recognized.
- Reproductive Planning: Stop ZEPBOUND® at least 2 months before a planned pregnancy due to its long half-life.
- Oral Contraceptives: ZEPBOUND® may affect the efficacy of oral birth control. Women should switch to a non-oral contraceptive method, or add a barrier method for 4 weeks after starting treatment and after each dose escalation.
To Report Adverse Reactions, contact the FDA:
- By phone: 1-800-FDA-1088
- Online: www.fda.gov/medwatch
Disclaimer
These statements are intended for informational purposes and do not replace professional medical advice. Talk to your healthcare provider about any questions or concerns related to ZEPBOUND®.
Note: The above statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Frequently Asked Questions
What are the side effects of Zepbound?
Potential side-effects can vary between individuals, based on medical history and lifestyle factors. The most common side effects are gastrointestinal: nausea, vomiting, diarrhea, and constipation. These often occur during dose titration and typically decrease over time. Rare but serious risks include pancreatitis and gallbladder issues.
How do I store Zepbound pens?
Store in the refrigerator between 36–46°F. Once in use, the pen may be stored at room temperature (up to 86°F) for up to 56 days.
Additional support
For details about your medication and dosing, please log in to your patient portal. Your physician is here to help with any medical questions. If you are experiencing a medical emergency, call 911 right away.